Anaerobic Cellular Respiration
- The Key Enzyme That Generates Ethanol During Fermentation Is Used
- The Key Enzyme That Generates Ethanol During Fermentation Is A Protein
- The Key Enzyme That Generates Ethanol During Fermentation Is Called
- The Key Enzyme That Generates Ethanol During Fermentation Is A Water
- The Key Enzyme That Generates Ethanol During Fermentation Is Done
- The Key Enzyme That Generates Ethanol During Fermentation Is Done
- The key point is to appreciate that fermentation is a broad term not solely associated with the conversion of pyruvate to lactic acid or ethanol. Source: Original work Marc T. Facciotti A note on the link between substrate level phosphorylation and fermentation.
- Ethanol Production by Enzymatic Hydrolysis Parametric Analysis of a Base-CaseProcess Steven H. Isaacs May 1984 Prepared under Task No. 1372.35 Solar Energy Research Institute A Division of Midwest Research Institute 1617 Cole Boulevard Golden, Colorado 80401 Prepared for the U.S. Department of Energy Contract No.
- Corn goes into the plant and is broken down by enzymes to prepare it for fermentation. During fermentation, yeast is added. The yeast consumes the raw materials and releases ethanol and carbon dioxide. Ethanol producers spend a lot of time and energy ensuring that the right conditions exist for yeast to thrive.
Ethanol fermentation is a two-step process. Pyruvate (pyruvic acid) is first converted into carbon dioxide and acetaldehyde. The second step converts. Jan 31, 2020 During beer production, yeast generate ethanol that is exported to the extracellular environment where it accumulates. Depending on the initial carbohydrate concentration in the wort, the amount of yeast biomass inoculated, the fermentation temperature, and the yeast attenuation capacity, a high concentration of ethanol can be achieved in beer. The increase in ethanol concentration as a.
Some prokaryotes and eukaryotes use anaerobic respiration in which they can create energy for use in the absence of oxygen.
Learning Objectives
Describe the process of anaerobic cellular respiration.
Key Takeaways
Key Points
- Anaerobic respiration is a type of respiration where oxygen is not used; instead, organic or inorganic molecules are used as final electron acceptors.
- Fermentation includes processes that use an organic molecule to regenerate NAD+ from NADH.
- Types of fermentation include lactic acid fermentation and alcohol fermentation, in which ethanol is produced.
- All forms of fermentation except lactic acid fermentation produce gas, which plays a role in the laboratory identification of bacteria.
- Some types of prokaryotes are facultatively anaerobic, which means that they can switch between aerobic respiration and fermentation, depending on the availability of oxygen.
Key Terms
- archaea: A group of single-celled microorganisms. They have no cell nucleus or any other membrane-bound organelles within their cells.
- anaerobic respiration: A form of respiration using electron acceptors other than oxygen.
- fermentation: An anaerobic biochemical reaction. When this reaction occurs in yeast, enzymes catalyze the conversion of sugars to alcohol or acetic acid with the evolution of carbon dioxide.
Anaerobic Cellular Respiration
The production of energy requires oxygen. The electron transport chain, where the majority of ATP is formed, requires a large input of oxygen. However, many organisms have developed strategies to carry out metabolism without oxygen, or can switch from aerobic to anaerobic cell respiration when oxygen is scarce.
During cellular respiration, some living systems use an organic molecule as the final electron acceptor. Processes that use an organic molecule to regenerate NAD+ from NADH are collectively referred to as fermentation. In contrast, some living systems use an inorganic molecule as a final electron acceptor. Both methods are called anaerobic cellular respiration, where organisms convert energy for their use in the absence of oxygen.
Certain prokaryotes, including some species of bacteria and archaea, use anaerobic respiration. For example, the group of archaea called methanogens reduces carbon dioxide to methane to oxidize NADH. These microorganisms are found in soil and in the digestive tracts of ruminants, such as cows and sheep. Similarly, sulfate-reducing bacteria and archaea, most of which are anaerobic, reduce sulfate to hydrogen sulfide to regenerate NAD+ from NADH.
Anaerobic bacteria: The green color seen in these coastal waters is from an eruption of hydrogen sulfide-producing bacteria. These anaerobic, sulfate-reducing bacteria release hydrogen sulfide gas as they decompose algae in the water.
Eukaryotes can also undergo anaerobic respiration. Some examples include alcohol fermentation in yeast and lactic acid fermentation in mammals.
Lactic Acid Fermentation
The fermentation method used by animals and certain bacteria (like those in yogurt) is called lactic acid fermentation. This type of fermentation is used routinely in mammalian red blood cells and in skeletal muscle that has an insufficient oxygen supply to allow aerobic respiration to continue (that is, in muscles used to the point of fatigue). The excess amount of lactate in those muscles is what causes the burning sensation in your legs while running. This pain is a signal to rest the overworked muscles so they can recover. In these muscles, lactic acid accumulation must be removed by the blood circulation and the lactate brought to the liver for further metabolism. The chemical reactions of lactic acid fermentation are the following:
Pyruvic acid + NADH ↔ lactic acid + NAD+
Lactic acid fermentation: Lactic acid fermentation is common in muscle cells that have run out of oxygen.
The enzyme used in this reaction is lactate dehydrogenase (LDH). The reaction can proceed in either direction, but the reaction from left to right is inhibited by acidic conditions. Such lactic acid accumulation was once believed to cause muscle stiffness, fatigue, and soreness, although more recent research disputes this hypothesis. Once the lactic acid has been removed from the muscle and circulated to the liver, it can be reconverted into pyruvic acid and further catabolized for energy.
Alcohol Fermentation
Another familiar fermentation process is alcohol fermentation, which produces ethanol, an alcohol. The use of alcohol fermentation can be traced back in history for thousands of years. The chemical reactions of alcoholic fermentation are the following (Note: CO2 does not participate in the second reaction):
Dec 28, 2016 Guild Wars 2: Heart of Thorns Serial Key Download Code Crack key generator Full Game Torrent skidrow Origin Key and Steam Online Code Avaiable. Guild Wars 2: Heart of Thorns Serial Key Cd Key Free Download Crack Full Game Guild Wars 2: Heart of Thorns Serial Cd Key Generator License Activator Product Origin Keys Full Game Download Free. Guild Wars 2: Heart of Thorns Keygen is here and it is FREE and 100% working and legit. Before our system send cd key, you will need to pass this human verification step. In order to bypass this step, you will need to complete a short and simple offer. Feb 10, 2020 Guild Wars 2 Heart of Thorns Digital Deluxe PC License Key MOBA matches motivated the stronghold and has been developed to provide players with a new spin on PvP. In this game mode, players will need to break through defences and enemy lines to get rid of the lord of the team. May 01, 2017 Thanks to this fantastic Guild Wars 2: Heart of Thorns Generator you can generate different Keys for you and your friends!The only Guild Wars 2: Heart of Thorns code generator that works.No download required.We just released a new leaked Guild Wars 2: Heart of Thorns Serial Key Generator that can generate keys for Windows PC, Xbox One. Guild wars 2 heart of thorns key generator. Guild Wars 2: Heart of Thorns is the first expansion for Guild Wars 2. Founded on the idea that the journey is the goal, the Heart of Thorns expansion continues the Guild Wars 2 tradition of challenging the conventions of MMOs to fulfill the promise of what online worlds should be.
Pyruvic acid → CO2 + acetaldehyde + NADH → ethanol + NAD+
Alcohol Fermentation: Fermentation of grape juice into wine produces CO2 as a byproduct. Fermentation tanks have valves so that the pressure inside the tanks created by the carbon dioxide produced can be released.
The first reaction is catalyzed by pyruvate decarboxylase, a cytoplasmic enzyme, with a coenzyme of thiamine pyrophosphate (TPP, derived from vitamin B1 and also called thiamine). A carboxyl group is removed from pyruvic acid, releasing carbon dioxide as a gas. The loss of carbon dioxide reduces the size of the molecule by one carbon, making acetaldehyde. The second reaction is catalyzed by alcohol dehydrogenase to oxidize NADH to NAD+ and reduce acetaldehyde to ethanol.
The Key Enzyme That Generates Ethanol During Fermentation Is Used
The fermentation of pyruvic acid by yeast produces the ethanol found in alcoholic beverages. Ethanol tolerance of yeast is variable, ranging from about 5 percent to 21 percent, depending on the yeast strain and environmental conditions.
Other Types of Fermentation
The Key Enzyme That Generates Ethanol During Fermentation Is A Protein
Various methods of fermentation are used by assorted organisms to ensure an adequate supply of NAD+ for the sixth step in glycolysis. Without these pathways, that step would not occur and no ATP would be harvested from the breakdown of glucose.Other fermentation methods also occur in bacteria. Many prokaryotes are facultatively anaerobic. This means that they can switch between aerobic respiration and fermentation, depending on the availability of oxygen. Certain prokaryotes, like Clostridia, are obligate anaerobes. Obligate anaerobes live and grow in the absence of molecular oxygen. Oxygen is a poison to these microorganisms, killing them on exposure.
It should be noted that all forms of fermentation, except lactic acid fermentation, produce gas. The production of particular types of gas is used as an indicator of the fermentation of specific carbohydrates, which plays a role in the laboratory identification of the bacteria.
Learning Objectives
- Define fermentation and explain why it does not require oxygen
- Describe the fermentation pathways and their end products and give examples of microorganisms that use these pathways
- Compare and contrast fermentation and anaerobic respiration
Many cells are unable to carry out respiration because of one or more of the following circumstances:
- The cell lacks a sufficient amount of any appropriate, inorganic, final electron acceptor to carry out cellular respiration.
- The cell lacks genes to make appropriate complexes and electron carriers in the electron transport system.
- The cell lacks genes to make one or more enzymes in the Krebs cycle.
Whereas lack of an appropriate inorganic final electron acceptor is environmentally dependent, the other two conditions are genetically determined. Thus, many prokaryotes, including members of the clinically important genus Streptococcus, are permanently incapable of respiration, even in the presence of oxygen. Conversely, many prokaryotes are facultative, meaning that, should the environmental conditions change to provide an appropriate inorganic final electron acceptor for respiration, organisms containing all the genes required to do so will switch to cellular respiration for glucose metabolism because respiration allows for much greater ATP production per glucose molecule.
If respiration does not occur, NADH must be reoxidized to NAD+ for reuse as an electron carrier for glycolysis, the cell’s only mechanism for producing any ATP, to continue. Some living systems use an organic molecule (commonly pyruvate) as a final electron acceptor through a process called fermentation. Fermentation does not involve an electron transport system and does not directly produce any additional ATP beyond that produced during glycolysis by substrate-level phosphorylation. Organisms carrying out fermentation, called fermenters, produce a maximum of two ATP molecules per glucose during glycolysis. Table 1 compares the final electron acceptors and methods of ATP synthesis in aerobic respiration, anaerobic respiration, and fermentation. Note that the number of ATP molecules shown for glycolysis assumes the Embden-Meyerhof-Parnas pathway. The number of ATP molecules made by substrate-level phosphorylation (SLP) versus oxidative phosphorylation (OP) are indicated.
| Table 1. Comparison of Respiration Versus Fermentation | ||||
|---|---|---|---|---|
| Type of Metabolism | Example | Final Electron Acceptor | Pathways Involved in ATP Synthesis (Type of Phosphorylation) | Maximum Yield of ATP Molecules |
| Aerobic respiration | Pseudomonas aeruginosa | [latex]{text{O}}_{2}[/latex] | EMP glycolysis (SLP) Krebs cycle (SLP) Electron transport and chemiosmosis (OP): | 2 2 34 |
| Total | 38 | |||
| Anaerobic respiration | Paracoccus denitrificans | [latex]{{text{NO}}_{3}}^{-},{text{SO}}_{4}^{-2},{text{Fe}}^{+3},{text{CO}}_{2}[/latex], other inorganics | EMP glycolysis (SLP) Krebs cycle (SLP) Electron transport and chemiosmosis (OP): | 2 2 1–32 |
| Total | 5–36 | |||
| Fermentation | Candida albicans | Organics (usually pyruvate) | EMP glycolysis (SLP) Fermentation | 2 0 |
| Total | 2 | |||
Microbial fermentation processes have been manipulated by humans and are used extensively in the production of various foods and other commercial products, including pharmaceuticals. Microbial fermentation can also be useful for identifying microbes for diagnostic purposes.
Fermentation by some bacteria, like those in yogurt and other soured food products, and by animals in muscles during oxygen depletion, is lactic acid fermentation. The chemical reaction of lactic acid fermentation is as follows:
[latex]text{Pyruvate + NADH}text{ }stackrel{}{leftrightarrow }text{ }{text{lactic acid + NAD}}^{text{+}}[/latex]
Bacteria of several gram-positive genera, including Lactobacillus, Leuconostoc, and Streptococcus, are collectively known as the lactic acid bacteria (LAB), and various strains are important in food production. During yogurt and cheese production, the highly acidic environment generated by lactic acid fermentation denatures proteins contained in milk, causing it to solidify. When lactic acid is the only fermentation product, the process is said to be homolactic fermentation; such is the case for Lactobacillus delbrueckii and S. thermophiles used in yogurt production. However, many bacteria perform heterolactic fermentation, producing a mixture of lactic acid, ethanol and/or acetic acid, and CO2 as a result, because of their use of the branched pentose phosphate pathway instead of the EMP pathway for glycolysis. One important heterolactic fermenter is Leuconostoc mesenteroides, which is used for souring vegetables like cucumbers and cabbage, producing pickles and sauerkraut, respectively.
Lactic acid bacteria are also important medically. The production of low pH environments within the body inhibits the establishment and growth of pathogens in these areas. For example, the vaginal microbiota is composed largely of lactic acid bacteria, but when these bacteria are reduced, yeast can proliferate, causing a yeast infection. Additionally, lactic acid bacteria are important in maintaining the health of the gastrointestinal tract and, as such, are the primary component of probiotics.
Another familiar fermentation process is alcohol fermentation, which produces ethanol. The ethanol fermentation reaction is shown in Figure 1. In the first reaction, the enzyme pyruvate decarboxylase removes a carboxyl group from pyruvate, releasing CO2 gas while producing the two-carbon molecule acetaldehyde. The second reaction, catalyzed by the enzyme alcohol dehydrogenase, transfers an electron from NADH to acetaldehyde, producing ethanol and NAD+. The ethanol fermentation of pyruvate by the yeast Saccharomyces cerevisiae is used in the production of alcoholic beverages and also makes bread products rise due to CO2 production. Outside of the food industry, ethanol fermentation of plant products is important in biofuel production.
Figure 1. The chemical reactions of alcohol fermentation are shown here. Ethanol fermentation is important in the production of alcoholic beverages and bread.
Beyond lactic acid fermentation and alcohol fermentation, many other fermentation methods occur in prokaryotes, all for the purpose of ensuring an adequate supply of NAD+ for glycolysis (Table 2). Without these pathways, glycolysis would not occur and no ATP would be harvested from the breakdown of glucose. It should be noted that most forms of fermentation besides homolactic fermentation produce gas, commonly CO2 and/or hydrogen gas. Many of these different types of fermentation pathways are also used in food production and each results in the production of different organic acids, contributing to the unique flavor of a particular fermented food product. The propionic acid produced during propionic acid fermentation contributes to the distinctive flavor of Swiss cheese, for example.
Several fermentation products are important commercially outside of the food industry. For example, chemical solvents such as acetone and butanol are produced during acetone-butanol-ethanol fermentation. Complex organic pharmaceutical compounds used in antibiotics (e.g., penicillin), vaccines, and vitamins are produced through mixed acid fermentation. Fermentation products are used in the laboratory to differentiate various bacteria for diagnostic purposes. For example, enteric bacteria are known for their ability to perform mixed acid fermentation, reducing the pH, which can be detected using a pH indicator. Similarly, the bacterial production of acetoin during butanediol fermentation can also be detected. Gas production from fermentation can also be seen in an inverted Durham tube that traps produced gas in a broth culture.
Microbes can also be differentiated according to the substrates they can ferment. For example, E. coli can ferment lactose, forming gas, whereas some of its close gram-negative relatives cannot. The ability to ferment the sugar alcohol sorbitol is used to identify the pathogenic enterohemorrhagic O157:H7 strain of E. coli because, unlike other E. coli strains, it is unable to ferment sorbitol. Last, mannitol fermentation differentiates the mannitol-fermenting Staphylococcus aureus from other non–mannitol-fermenting staphylococci.
| Table 2. Common Fermentation Pathways | |||
|---|---|---|---|
| Pathway | End Products | Example Microbes | Commercial Products |
| Acetone-butanol-ethanol | Acetone, butanol, ethanol, CO2 | Clostridium acetobutylicum | Commercial solvents, gasoline alternative |
| Alcohol | Ethanol, CO2 | Candida, Saccharomyces | Beer, bread |
| Butanediol | Formic and lactic acid; ethanol; acetoin; 2,3 butanediol; CO2; hydrogen gas | Klebsiella, Enterobacter | Chardonnay wine |
| Butyric acid | Butyric acid, CO2, hydrogen gas | Clostridium butyricum | Butter |
| Lactic acid | Lactic acid | Streptococcus, Lactobacillus | Sauerkraut, yogurt, cheese |
| Mixed acid | Acetic, formic, lactic, and succinic acids; ethanol, CO2, hydrogen gas | Escherichia, Shigella | Vinegar, cosmetics, pharmaceuticals |
| Propionic acid | Acetic acid, propionic acid, CO2 | Propionibacterium, Bifidobacterium | Swiss cheese |
Think about It
- When would a metabolically versatile microbe perform fermentation rather than cellular respiration?
Identifying Bacteria by Using API Test Panels
Identification of a microbial isolate is essential for the proper diagnosis and appropriate treatment of patients. Scientists have developed techniques that identify bacteria according to their biochemical characteristics. Typically, they either examine the use of specific carbon sources as substrates for fermentation or other metabolic reactions, or they identify fermentation products or specific enzymes present in reactions. In the past, microbiologists have used individual test tubes and plates to conduct biochemical testing. However, scientists, especially those in clinical laboratories, now more frequently use plastic, disposable, multitest panels that contain a number of miniature reaction tubes, each typically including a specific substrate and pH indicator. After inoculation of the test panel with a small sample of the microbe in question and incubation, scientists can compare the results to a database that includes the expected results for specific biochemical reactions for known microbes, thus enabling rapid identification of a sample microbe. These test panels have allowed scientists to reduce costs while improving efficiency and reproducibility by performing a larger number of tests simultaneously.
Many commercial, miniaturized biochemical test panels cover a number of clinically important groups of bacteria and yeasts. One of the earliest and most popular test panels is the Analytical Profile Index (API) panel invented in the 1970s. Once some basic laboratory characterization of a given strain has been performed, such as determining the strain’s Gram morphology, an appropriate test strip that contains 10 to 20 different biochemical tests for differentiating strains within that microbial group can be used. Currently, the various API strips can be used to quickly and easily identify more than 600 species of bacteria, both aerobic and anaerobic, and approximately 100 different types of yeasts. Based on the colors of the reactions when metabolic end products are present, due to the presence of pH indicators, a metabolic profile is created from the results (Figure 2). Microbiologists can then compare the sample’s profile to the database to identify the specific microbe.
Figure 2. The API 20NE test strip is used to identify specific strains of gram-negative bacteria outside the Enterobacteriaceae. Here is an API 20NE test strip result for Photobacterium damselae ssp. piscicida.
Clinical Focus: Alex, Part 2
This example continues Alex’s story that started in Energy Matter and Enzymes.
Many of Alex’s symptoms are consistent with several different infections, including influenza and pneumonia. However, his sluggish reflexes along with his light sensitivity and stiff neck suggest some possible involvement of the central nervous system, perhaps indicating meningitis. Meningitis is an infection of the cerebrospinal fluid (CSF) around the brain and spinal cord that causes inflammation of the meninges, the protective layers covering the brain. Meningitis can be caused by viruses, bacteria, or fungi. Although all forms of meningitis are serious, bacterial meningitis is particularly serious. Bacterial meningitis may be caused by several different bacteria, but the bacterium Neisseria meningitidis, a gram-negative, bean-shaped diplococcus, is a common cause and leads to death within 1 to 2 days in 5% to 10% of patients.
Given the potential seriousness of Alex’s conditions, his physician advised his parents to take him to the hospital in the Gambian capital of Banjul and there have him tested and treated for possible meningitis. After a 3-hour drive to the hospital, Alex was immediately admitted. Physicians took a blood sample and performed a lumbar puncture to test his CSF. They also immediately started him on a course of the antibiotic ceftriaxone, the drug of choice for treatment of meningitis caused by N. meningitidis, without waiting for laboratory test results.
- How might biochemical testing be used to confirm the identity of N. meningitidis?
- Why did Alex’s doctors decide to administer antibiotics without waiting for the test results?
We’ll return to Alex’s example in later pages.
Key Concepts and Summary
- Fermentation uses an organic molecule as a final electron acceptor to regenerate NAD+ from NADH so that glycolysis can continue.
- Fermentation does not involve an electron transport system, and no ATP is made by the fermentation process directly. Fermenters make very little ATP—only two ATP molecules per glucose molecule during glycolysis.
- Microbial fermentation processes have been used for the production of foods and pharmaceuticals, and for the identification of microbes.
- During lactic acid fermentation, pyruvate accepts electrons from NADH and is reduced to lactic acid. Microbes performing homolactic fermentation produce only lactic acid as the fermentation product; microbes performing heterolactic fermentation produce a mixture of lactic acid, ethanol and/or acetic acid, and CO2.
- Lactic acid production by the normal microbiota prevents growth of pathogens in certain body regions and is important for the health of the gastrointestinal tract.
- During ethanol fermentation, pyruvate is first decarboxylated (releasing CO2) to acetaldehyde, which then accepts electrons from NADH, reducing acetaldehyde to ethanol. Ethanol fermentation is used for the production of alcoholic beverages, for making bread products rise, and for biofuel production.
- Fermentation products of pathways (e.g., propionic acid fermentation) provide distinctive flavors to food products. Fermentation is used to produce chemical solvents (acetone-butanol-ethanol fermentation) and pharmaceuticals (mixed acid fermentation).
- Specific types of microbes may be distinguished by their fermentation pathways and products. Microbes may also be differentiated according to the substrates they are able to ferment.
Multiple Choice
Which of the following is the purpose of fermentation?
- to make ATP
- to make carbon molecule intermediates for anabolism
- to make NADH
- to make NAD+
Which molecule typically serves as the final electron acceptor during fermentation?
- oxygen
- NAD+
- pyruvate
- CO2
The Key Enzyme That Generates Ethanol During Fermentation Is Called
Show AnswerWhich fermentation product is important for making bread rise?
- ethanol
- CO2
- lactic acid
- hydrogen gas
Which of the following is not a commercially important fermentation product?
- ethanol
- pyruvate
- butanol
- penicillin
Fill in the Blank
The microbe responsible for ethanol fermentation for the purpose of producing alcoholic beverages is ________.
Show Answer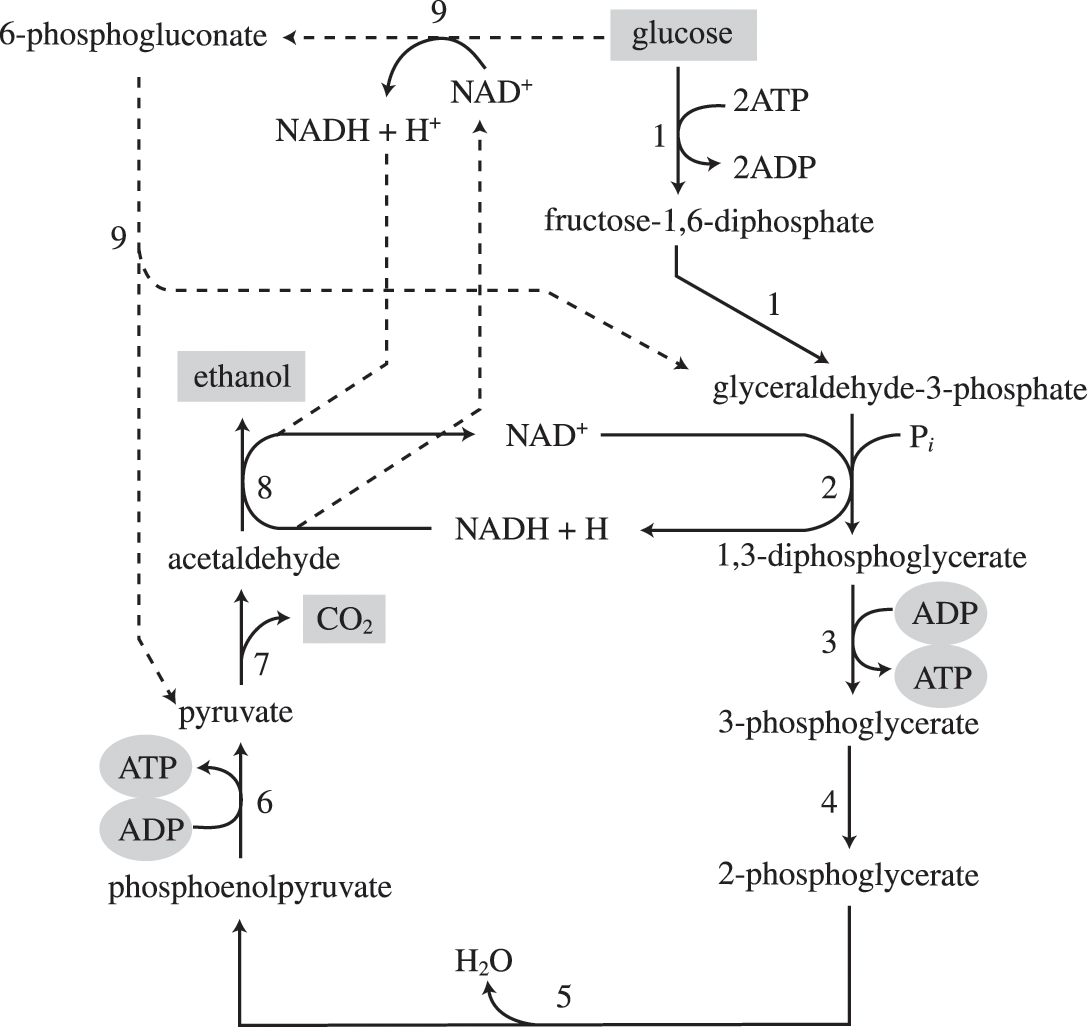
________ results in the production of a mixture of fermentation products, including lactic acid, ethanol and/or acetic acid, and CO2.
 Show Answer
Show AnswerThe Key Enzyme That Generates Ethanol During Fermentation Is A Water
Fermenting organisms make ATP through the process of ________.
Show AnswerThe Key Enzyme That Generates Ethanol During Fermentation Is Done
Matching
Match the fermentation pathway with the correct commercial product it is used to produce:
| ___acetone-butanol-ethanol fermentation | a. bread |
| ___alcohol fermentation | b. pharmaceuticals |
| ___lactic acid fermentation | c. Swiss cheese |
| ___mixed acid fermentation | d. yogurt |
| ___propionic acid fermentation | e. industrial solvents |
- Industrial solvents are produced by acetone-butanol-ethanol fermentation.
- Bread is produced by alcohol fermentation.
- Yogurt is produced by lactic acid fermentation.
- Pharmaceuticals are produced by mixed acid fermentation.
- Swiss cheese is produced by propionic acid fermentation.
The Key Enzyme That Generates Ethanol During Fermentation Is Done
Think about It
- Why are some microbes, including Streptococcus spp., unable to perform aerobic respiration, even in the presence of oxygen?
- How can fermentation be used to differentiate various types of microbes?
- The bacterium E. coli is capable of performing aerobic respiration, anaerobic respiration, and fermentation. When would it perform each process and why? How is ATP made in each case?